お伺い:非観血式で、観血血圧を解析できるアルゴリズムは、米国・カナダ・豪州・英国・欧州等に於いて認可されていますが、本邦に於いては、ハンガリ国テンシオメド社のアルテリオグラフ24に於いて一旦認可された仕様を途中で不可になった経験から、今回は、ラブテック社モバイル12誘導心電計に習い、研究会を開催させて頂き、日本の専門医のご検証賜ってから薬機法申請にしたいと考えております、
もし、認可申請が急がれる場合にはメディカルテクニカ宛ご指示下さい、
なお、本原理は、米国軍事中央研究所とバージニア大学が多数の特許と数か年及び数か国の検証を経て、2017年2020年に米国FDAに認可取得しております、
なお、メーカでは、デバイスご購入だけで、人工知能ソフト開発に無償でご参画頂くとの方針です、
睡眠時無呼吸の臨床試験にも当該パラメータがお役に立つものと日本からの高度な検証を期待しているとのお伺いが参っております、
心不全・血栓症・心筋炎、不整脈・高血圧・腎不全、脳梗塞、等遠隔でデータが連続で自動入手できる手段をご提供できるものと期待しております、
なお、当該心電図1チャンネルは充電式の為、エンドレスで何か月もデータを取ることが可能で、2015年に薬機法に認可されている製品です、
体温も充電式です、
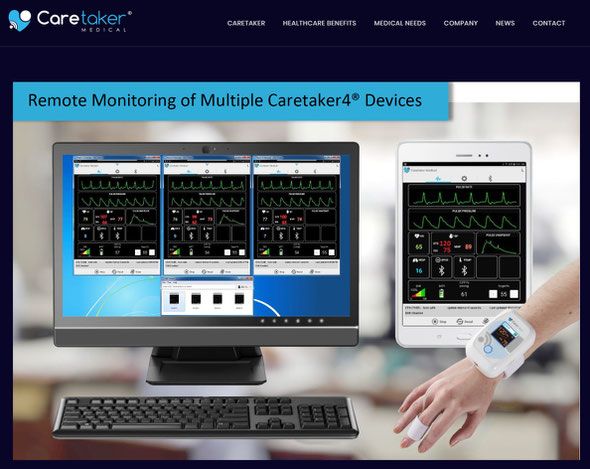
ディジタル最先端技術搭載ーインターネット技術のフル機能ー低価格
FDA認可ー非観血式で観血圧をモニター、長時間・連続・ウエアラブル
・解析出力・連続心拍出量・連続動脈硬化指標解析出力・心拍数・呼吸数
パルスオキㇱ・体温・長時間心電図・多人数同時システム
MRI下連続モニター可
2003年開発、2007年日本は研究用で納入開始
新型コロナの無症状、中等症、重症、後遺症及び診断・治療薬等の創薬
のご研究に世界が挑戦しており、お役に立てれれば幸いです


最新文献 MRI中の血圧変化の解析
httpswww.ncbi.nlm.nih.govpmcarticlesPMC5361833
BMC Anesthesiol. 2017; 17: 48.
Published online 2017 Mar 21. doi: 10.1186/s12871-017-0337-z
PMCID: PMC5361833
PMID: 28327093
Continuous Non-invasive finger cuff CareTaker® comparable to invasive intra-arterial pressure in patients undergoing major intra-abdominal surgery
Irwin Gratz,1 Edward Deal,1 Francis Spitz,1 Martin Baruch,2 I. Elaine Allen,3 Julia E. Seaman,4 Erin Pukenas,1 and Smith Jean1
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Associated Data
Data Availability Statement
The datasets generated during and analysed for the current study are available from the corresponding author on reasonable request.
Abstract
Background
Despite increased interest in non-invasive arterial pressure monitoring, the majority of commercially available technologies have failed to satisfy the limits established for the validation of automatic arterial pressure monitoring by the Association for the Advancement of Medical Instrumentation (AAMI). According to the ANSI/AAMI/ISO 81060–2:2013 standards, the group-average accuracy and precision are defined as acceptable if bias is not greater than 5 mmHg and standard deviation is not greater than 8 mmHg. In this study, these standards are used to evaluate the CareTaker® (CT) device, a device measuring continuous non-invasive blood pressure via a pulse contour algorithm called Pulse Decomposition Analysis.
Methods
A convenience sample of 24 patients scheduled for major abdominal surgery were consented to participate in this IRB approved pilot study. Each patient was monitored with a radial arterial catheter and CT using a finger cuff applied to the contralateral thumb. Hemodynamic variables were measured and analyzed from both devices for the first thirty minutes of the surgical procedure including the induction of anesthesia. The mean arterial pressure (MAP), systolic and diastolic blood pressures continuously collected from the arterial catheter and CT were compared. Pearson correlation coefficients were calculated between arterial catheter and CT blood pressure measurements, a Bland-Altman analysis, and polar and 4Q plots were created.
Results
The correlation of systolic, diastolic, and mean arterial pressures were 0.92, 0.86, 0.91, respectively (p < 0.0001 for all the comparisons). The Bland-Altman comparison yielded a bias (as measured by overall mean difference) of −0.57, −2.52, 1.01 mmHg for systolic, diastolic, and mean arterial pressures, respectively with a standard deviation of 7.34, 6.47, 5.33 mmHg for systolic, diastolic, and mean arterial pressures, respectively (p < 0.001 for all comparisons). The polar plot indicates little bias between the two methods (90%/95% CI at 31.5°/52°, respectively, overall bias = 1.5°) with only a small percentage of points outside these lines. The 4Q plot indicates good concordance and no bias between the methods.
Conclusions
In this study, blood pressure measured using the non-invasive CT device was shown to correlate well with the arterial catheter measurements. Larger studies are needed to confirm these results in more varied settings. Most patients exhibited very good agreement between methods. Results were well within the limits established for the validation of automatic arterial pressure monitoring by the AAMI.
Keywords: Non-Invasive, CareTaker, Central blood pressure, Finger cuff, Intra-Arterial pressure
Go to:
Background
Accurate real-time continuous non-invasive blood pressure monitors (cNIBP) can bridge the gap between invasive arterial pressure monitoring and intermittent non-invasive sphygmomanometry. Latest developments in this field promise accuracy and the potential to lower risk and improve patient outcomes. However, a recent systematic review and meta-analysis of 28 studies using non-invasive technologies by Kim et al. reported that all failed to satisfy the limits that have been established for the validation of automatic arterial pressure monitoring by the Association for the Advancement of Medical Instrumentation (AAMI) [1]. According to this standard, the group-average accuracy and precision are defined as acceptable if bias is not greater than 5 mmHg and standard deviation is not greater than 8 mmHg. Kim et.al. obtained similar results when currently commercially available technologies were examined [1]. In addition, ease of use and patient comfort issues have been impediments to wider acceptance of current noninvasive cNIBP measurement methods. Their results suggest that currently available devices may not have the accuracy and precision for reliable clinical decisions, and there is a need for better devices.
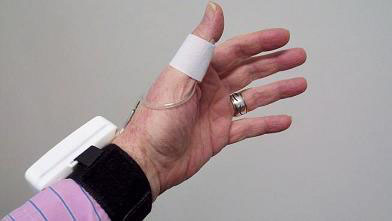
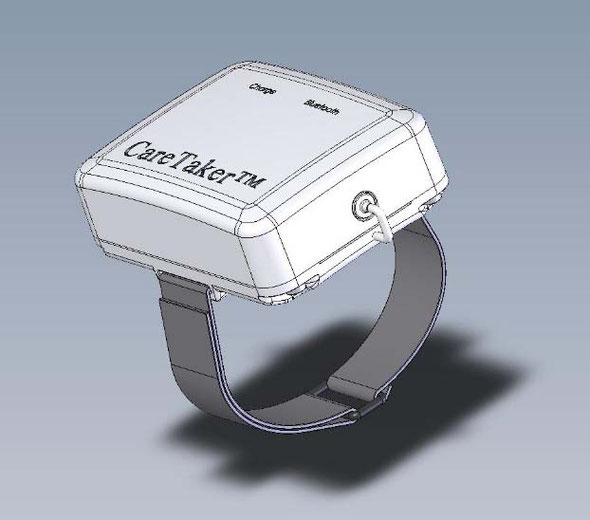
We evaluated the CareTaker® (CT) device (Empirical Technologies Corporation, Charlottesville, Virginia) which has been described in detail elsewhere [2]. Briefly, the CT is a physiological sensing system that communicates physiological data wirelessly via Bluetooth (Fig. 1). The device uses a low pressure [35–45 mmHg], pump-inflated, cuff surrounding the proximal phalange of the thumb that pneumatically couples arterial pulsations via a pressure line to a custom-designed piezo-electric pressure sensor. This sensor converts the pressure pulsations, using transimpedance amplification, into a derivative voltage signal that is then digitized at 500 Hz, transmitted to and recorded on a computer.
The CT measures continuous noninvasive blood pressure via a pulse contour analysis algorithm called Pulse Decomposition Analysis (PDA) [3]. It is based on the concept that five individual component pressure pulses constitute the peripheral arterial pressure pulse. These component pulses are due to the left ventricular ejection and the reflections and re-reflections of the first component pulse from two central arteries reflection sites [2] [4]. The first reflection site is the juncture between thoracic and abdominal aorta, at the height of the renal arteries, while the second site arises from the interface between abdominal aorta and the common iliac arteries. The renal site reflects the pressure pulse because the juncture of the aortic arteries there features significant changes in arterial diameter and wall elasticity. The two reflected arterial component pressure pulses, the renal reflection pulse (P2) and the iliac reflection pulse (P3), counter-propagate with respect to the original pulse due to the left ventricular contraction (Fig. 2) and arrive in the arterial periphery, specifically at the radial or digital arteries, with distinct time delays [5]. The basic validity of the PDA model was recently corroborated in a detailed and comprehensive arterial tree numerical modeling analysis [6] that examined the effect of the different arterial segments of the central arteries, the iliac arteries and beyond on the pressure/flow pulse patterns in the digital arteries. The results clearly identified the central arterial reflection sites, as opposed to more distal sites, as being the primary contributors to the pulse patterns observed in the digits.
Quantification and validation of physiological parameters is accomplished by extracting pertinent component pulse parameters [7]. Since the device relies on pulse analysis to track blood pressure, the coupling pressure of the finger cuff is maintained constant and well below diastole, avoiding potential blood flow impediments.
The aim of the present study was to specifically compare the non-invasive arterial pressure values obtained with the CT to the reference invasive arterial pressure technique.
Go to:
Methods
The Cooper Health System Institutional Review Board approved the study, and all subjects gave informed written consent. Data from twenty-four adult patients requiring hemodynamic monitoring during major open abdominal surgery were analyzed in this study. Patients were not excluded due to other medical conditions.
Measurements were obtained during general anesthesia in these patients starting with induction. The induction of anesthesia was chosen because the blood pressure fluctuations and variability typically found during this period provided an opportunity to compare tracking accuracy under baseline and induced controlled dynamic conditions. The data was evaluated using the ANSI/AAMI/ISO 81060–2:2013-related standards of accuracy and precision [8].
Anesthesia procedure
After a stable signal was recorded, patients were induced under general anesthesia by using propofol (2-4 mg/kg) and fentanyl 250ug. Tracheal intubation was facilitated by the administration of rocuronium (0.6 mg/kg). Mechanical ventilation was started using a volume controlled ventilator to maintain an adequate saturation and an end-tidal carbon dioxide of 35 mmHg. Inhalational anesthetic (Isoflurane) was added to maintain a BIS monitoring of 40–45. Vasoactive drugs were used to maintain a MAP greater than 60 mmHg based on the catheter value. Hemodynamic variables were measured from both devices for the entire procedure. The MAP, systolic and diastolic blood pressures were continuously collected from the arterial catheter and CT and averaged over 10 s periods for both devices.
Invasive arterial pressure measurement
Standard arterial blood pressure monitoring was performed prior to the induction of anesthesia using a 20G intra-arterial catheter inserted in the radial artery under local anesthesia using ultra sound guidance. The catheter was connected to a disposable pressure transducer with standard low compliant tubing. The transducer was placed at heart level and zeroed to ambient pressure. The transducer data was digitized, processed and collected using the Datex-Ohmeda S/5 Collect system (Datex-Ohmeda Division, Instrumentarium Corporation, Helsinki, Finland). For analysis, MAP, systolic and diastolic blood pressures were averaged over 10 s intervals.
Non-invasive CareTaker arterial pulse signal recording
The arterial pressure pulse signal was continuously measured using the CT device. For this study the CT device was calibrated using the arterial line blood pressure, but calibration can also be based on non-invasive oscillometric or oscillometric/auscultatory measurements. A fifteen second window at the start of the 30 min overlap section was used to obtain an arterial stiffness reading averaged across 5 beats, which was then used to calculate the PDA parameters for the blood pressure conversions (Fig. 2). With the exception of the four cases mentioned above, patient-specific PDA parameters, once established, were not changed for the matching procedure, irrespective of arterial stiffness or heart rate changes. On four occasions for the entire data set, the offsets of the linear conversion equations were changed as a result of persistent changes in arterial stiffness or heart rate changes exceeding 30%. The PDA algorithm has recently been validated and described elsewhere [6].
Data inclusion
Arterial catheter data were visually inspected and sections of obvious catheter failure, characterized by either continuous or spurious nonsensical reading, were excluded. Sections contaminated by excessive motion artifact such that the peak detection algorithm was no longer able to identify heart beats were also excluded. In the case of the CT data, a custom signal/noise factor (SNF) was used to identify poor quality data sections which were excluded. The factor is based on the standard ratio of the variances of the physiological signal band to the noise band and obtained using Fourier spectral analysis over an 8-s window with 1 s overlap [9]. The frequency range of the band associated with the physiological signal was set to 1–10 Hz, based on data by the authors and results by others, [7] while the noise band was set to the 100–250 Hz frequency range, which is subject to ambient noise but contains no signal relevant to the base band phenomena of the arterial pressure pulse or its propagation characteristics. Data sections with an SNF
below 80 were excluded from the analysis.


メディカルテクニカへようこそ マイクロセンサ
極めて簡単な方法で、生体現象を分析できる手段が開発されました。
それが、米国産 ワイヤレス 無線 連続 携帯移動式 ケアテイカです。
心拍数、相対血圧値、呼吸数の他数々の解析パラメータ
現在産業界の各種部品製品の生体融合設計の検討に欠かせません。
詳しくは下記サイトご参照
お問い合わせ メディカルテクニカ有限会社
電話048-928-0168 email medicalteknika@mail.goo.ne.jp まで
ワイヤレス ブルーツース 携帯 連続 非観血 血圧分析
詳しいご案内は、右のケアテイカをクリック願います
血管にカテーテルを差し込まないで、非観血式に、動脈波形を解析します。
一拍ごとに、周波数解析して、大動脈に駆出される血液の波形を解析します。
連続波形と連続血圧値を算出します。昔から指摘されていた反射波を解析します。
肝臓腎臓胃腸などから反射波と足の反射とを区別します。
ワイヤレスなどで、データ蓄積は相当量を無理なく記憶します。
すべての値はディジタル値で出力されます。
なお、測定上の一番気をつけなければならない点は
カフの空気圧がほぼ20から50mmHgの範囲で自動コントロールされる
ように、カフ内空気圧を落ち着くところまで設定してから測定に入ってください
詳細は右の項目をクリック願います



 スマホ スマートフォン スマートタブレット ワイヤレス モービル クラウドカーディオロジー12誘導心電計
スマホ スマートフォン スマートタブレット ワイヤレス モービル クラウドカーディオロジー12誘導心電計